LANGUAGE
PRINCIPLE
Electrochemical gas sensor
- Top
- Know the sensor
- Electrochemical gas sensor
What is an electrochemical gas sensor?
It is a sensor that specializes in detecting toxic gas.It has high selectivity and is used for various detection alarms for consumer and industrial use.

Features
- Due to the operating principle that does not consume power, it consumes less power and can be driven by batteries.
- Has high gas detection accuracy
- Since there are few mechanically weak parts, it is strong against drops, vibrations, impacts, etc.
- It is stable for a long time because it is hardly affected by poisoning such as silicon.
- Excellent reproducibility and stability because it is hardly affected by humidity
Basic structure of electrochemical gas sensor
The detection electrode, reference / counter electrode, electrolyte holder / separator, and air vent sheet are enclosed in a plastic case together with the electrolyte.Carbon monoxide passes through the capillary to remove miscellaneous gases in the spare chamber with a built-in charcoal filter, diffuses the PTFE sheet, reaches the detection electrode, and is oxidized there.The capillaries are also protected by a special filter that is dustproof and waterproof.
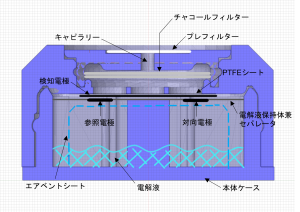
Detection principle of electrochemical gas sensor
The electrochemical gas sensor monitors the detection electrode where the oxidation (reduction) reaction occurs, the counter electrode where the reduction (oxidation) reaction occurs at the same time, and the potential change that occurs at the detection electrode at this time, and keeps the potential of the detection electrode constant. It consists of a reference electrode and keeps it.In the case of carbon monoxide detection, the following carbon monoxide oxidation reaction occurs at the detection electrode.
CO + H2O → CO2 + XNUMXH + + XNUMXe-
Here, when the detection electrode and the counter electrode are connected by an external circuit, electrons flow from the detection electrode toward the counter electrode, hydrogen ions move in the electrolytic solution, receive electrons on the counter electrode side, and react with oxygen to produce water. Will be generated.
2H + + O2 / XNUMX + XNUMXe- → HXNUMXO
In this way, the electrochemical gas sensor detects gas by converting the chemical reaction caused by redox into electrical energy.The state of these reactions is as shown in Fig. XNUMX, and the total reaction formula is
CO + O2 / 2 → COXNUMX
It is an oxidation reaction of carbon monoxide represented by.
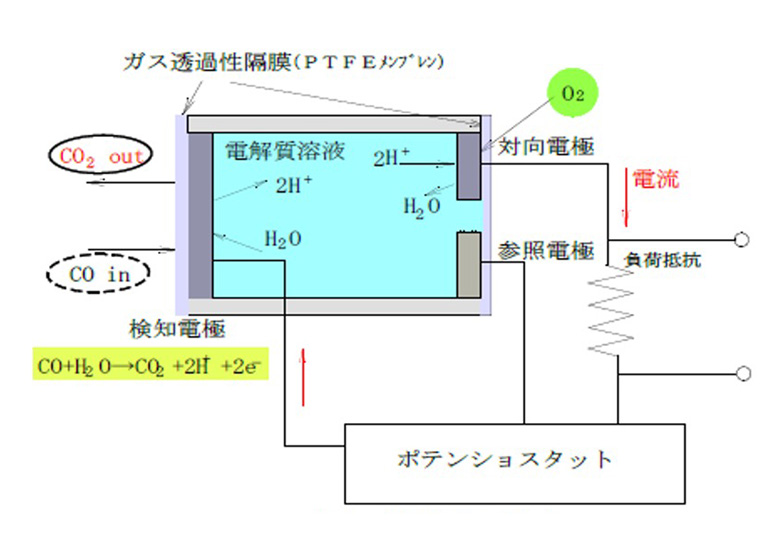
Figure 1: Operation principle diagram
In such a reaction process, a voltage drop occurs inside the electrolytic solution due to polarization caused by the reaction layer near the electrode and internal resistance that hydrogen ions receive when moving in the electrolytic solution.This voltage drop generally increases as the gas concentration increases, and acts as a factor that impairs the linearity of the output characteristics of the electrochemical gas sensor.Here, the effect of the reference electrode is that the potential of the detection electrode is detected, the potential of the detection electrode is kept constant regardless of this voltage drop, and a current proportional to the gas concentration always flows between the detection electrode and the counter electrode. make it possible.An electrochemical gas sensor with such a potential control function using a reference electrode is called a three-electrode method.Such a three-electrode method has been widely adopted in fields such as industrial gas concentration measurement because of its excellent linearity and stability.In contrast to the three-electrode method, the electrochemical gas sensor, which omits the reference electrode and consists of a detection electrode and a counter electrode, is called a two-electrode method.This method has been used for consumer gas alarms, etc., where accuracy is not required so much because of its cost advantage.Although it has the performance that can be used in consumer gas alarms, its linearity and output stability are inferior to those of the three-electrode method.
Main detection target gas
General toxic gases such as carbon monoxide, hydrogen sulfide, and ammonia





